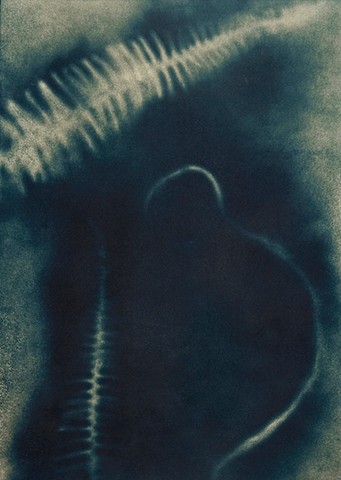
Gallery Two
Cyanotype (Blueprint or Ferro-Prussiate Process)
Sir John Hershel was the first to discover the photosensitivity of Ferric or iron salts (1842). He used the process to make copies of his notes, calculations, and botanical specimens. In the 1850s, photographer Henri Le Secq, made limited use of the process, and Anna Atkins, an English amateur working around the same time, produced numerous Cyanotypes of botanical specimens. Commercially prepared Cyanotype paper was available in the 1860s. It was during this period that engineers and draftsmen began using the process as a method of copying drawings and specifications.
The simplicity and low cost made Cyanotype popular with turn-of-the-century amateurs and professionals as a quick way to proof negatives without a darkroom. Use of Cyanotype has grown in the past generation among photographers and other artists eager to experiment with a printing process that is inexpensive and conducive to a wide range of manipulations and variations such as toning, hand coloring and without the necessity of using a photographic negative.
Cyanotype sensitizer can be made with Ferric Ammonium Citrate and Potassium Ferricyanide. The sensitizer is applied to paper, dried, and exposed to sunlight or UV light in contact with a “negative” that can be made photographically, hand drawn on a translucent surface or with tangible objects placed on the sensitized paper. After exposure, the Cyanotype is washed in water with Citric Acid to remove the unexposed iron salts (areas not affected by exposure). Upon drying, the image slowly oxidizes to a deep Prussian blue tone. The print may be changed to this deep blue tone immediately by immersing it in a dilute solution of hydrogen peroxide prior to washing.